Determination of binding characteristics and cytotoxic activity of bispecific T cell engagers
1 Quality Assistance S.A., Technoparc de Thudinie 2, 6536 Donstiennes, Belgium
2 Synaffix - A Lonza Company, Kloosterstraat 9, 5349 AB Oss, The Netherlands

1 Quality Assistance S.A., Technoparc de Thudinie 2, 6536 Donstiennes, Belgium
2 Synaffix - A Lonza Company, Kloosterstraat 9, 5349 AB Oss, The Netherlands
1 Quality Assistance
2 Waters Corporation

Adeno-associated viruses (AAV) have emerged as a leading vector for gene delivery for treating various diseases due to its safety profile and efficient transduction of numerous

Pyrogenic substances in pharmaceutical products can induce life threatening fever reactions. Endotoxins from Gram-negative bacteria were identified as the most prevalent and quantifiable pyrogens of concern.
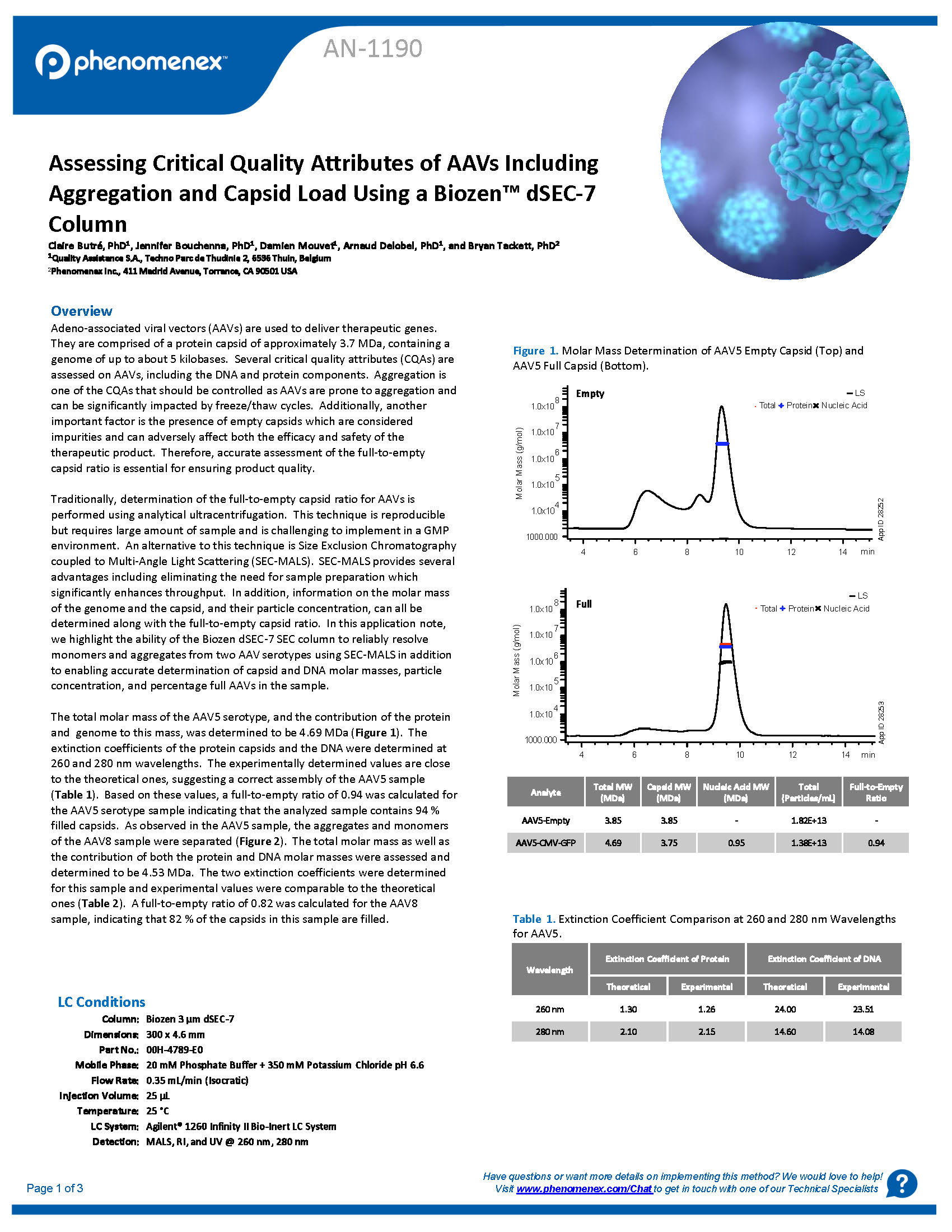
In this application note, we highlight the ability of the Biozen dSEC-7 SEC column to reliably resolve monomers and aggregates from two AAV serotypes using SEC-MALS in addition to enabling accurate determination of capsid and DNA molar masses, particle concentration, and percentage full AAVs in t

In the adaptive immune response, interactions between the T cell receptor (TCR) and the major histocompatibility complex (MHC) are crucial for immune system development, maturation, surveillance, and protection against pathogens and cancer. The primary function of the TCR is to r

Imaged capillary isoelectric focusing (icIEF) is the gold standard technique for the determination and relative quantification of biotherapeutics charge variants, considered as a critical quality attributes (CQA).

Peptide mapping is the key method for characterization of primary structure of biotherapeutic proteins. This method relies on digestion of proteins into peptides that are then analyzed for amino acid sequence and posttranslational modifications.
Since the discovery of effective methods of delivery, messenger RNAs (mRNAs) have become increasingly popular in healthcare. These large, complex molecules are designed to allow cells to produce critical proteins and enzymes that can help fight disease.

Therapeutic mRNA is receiving growing interest in various therapeutic applications such as genome editing, cancer immunotherapy and prophylactic vaccines.
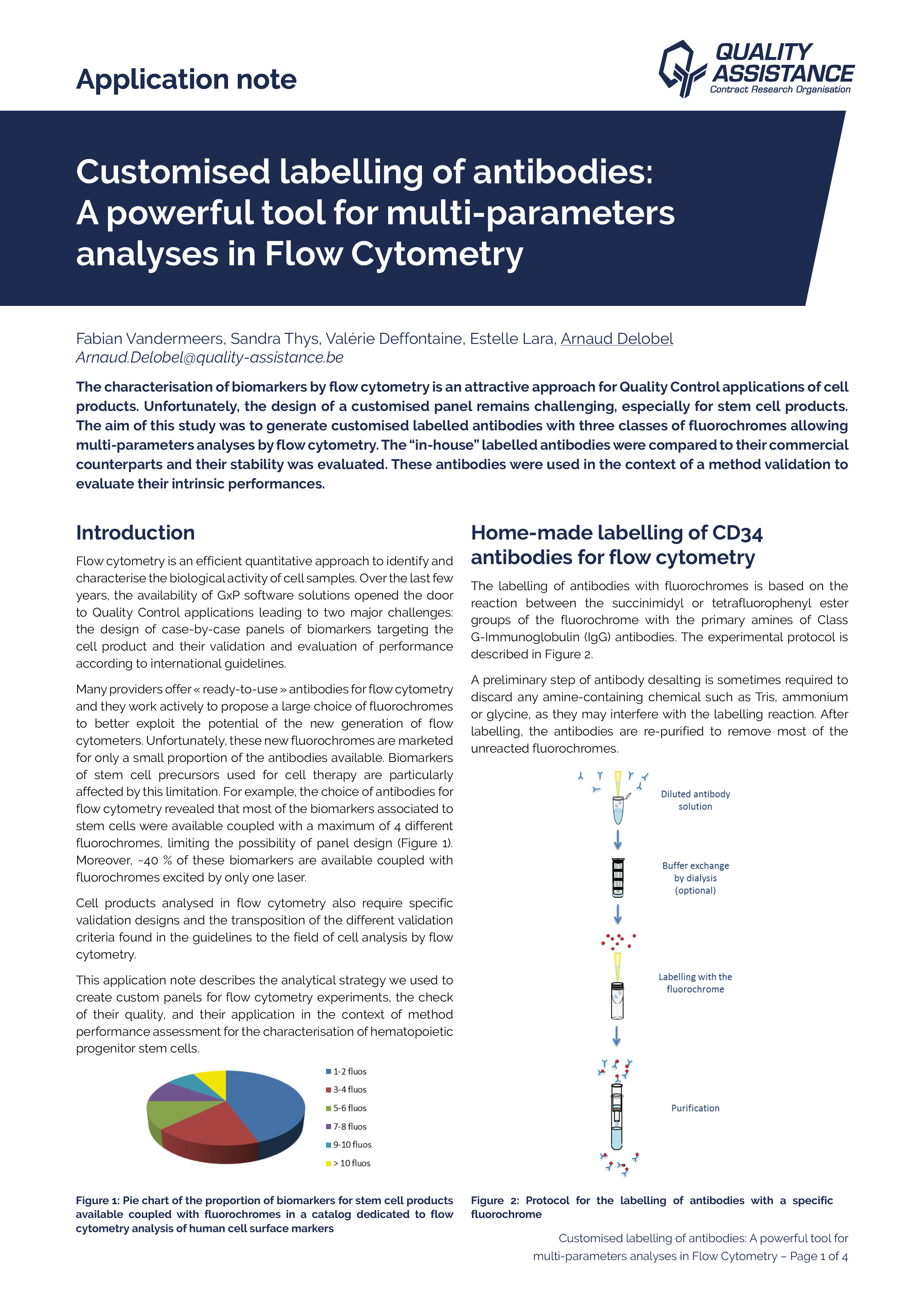
At the cutting edge of analytical sciences, Quality Assistance is constantly investing in the latest technologies and equipment to provide the pharmaceutical industry with up-to-date services required by regulatory au


Since 2015, Quality Assistance proposes to its partners a method based on Sulphur specific ICP-MS/MS detection combined with an 34S/32S isotope dilution quantification.

Adeno-associated viral vectors (AAVs) are small (~20-25 nm), non-enveloped viruses involved in the development of gene therapy protocols for the treatment of various diseases.

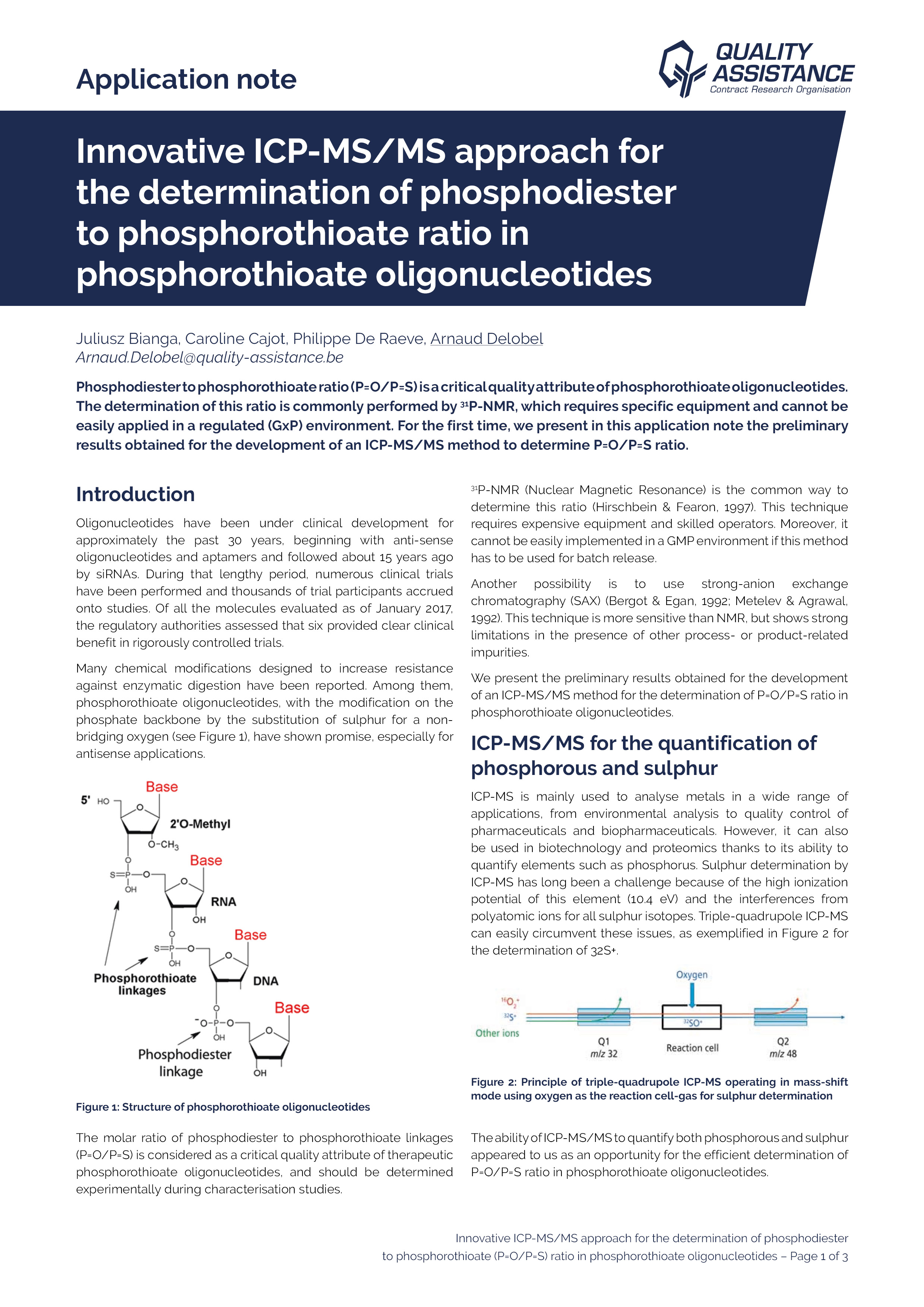
The field of therapeutic oligonucleotides has seen remarkable progress over the last years.
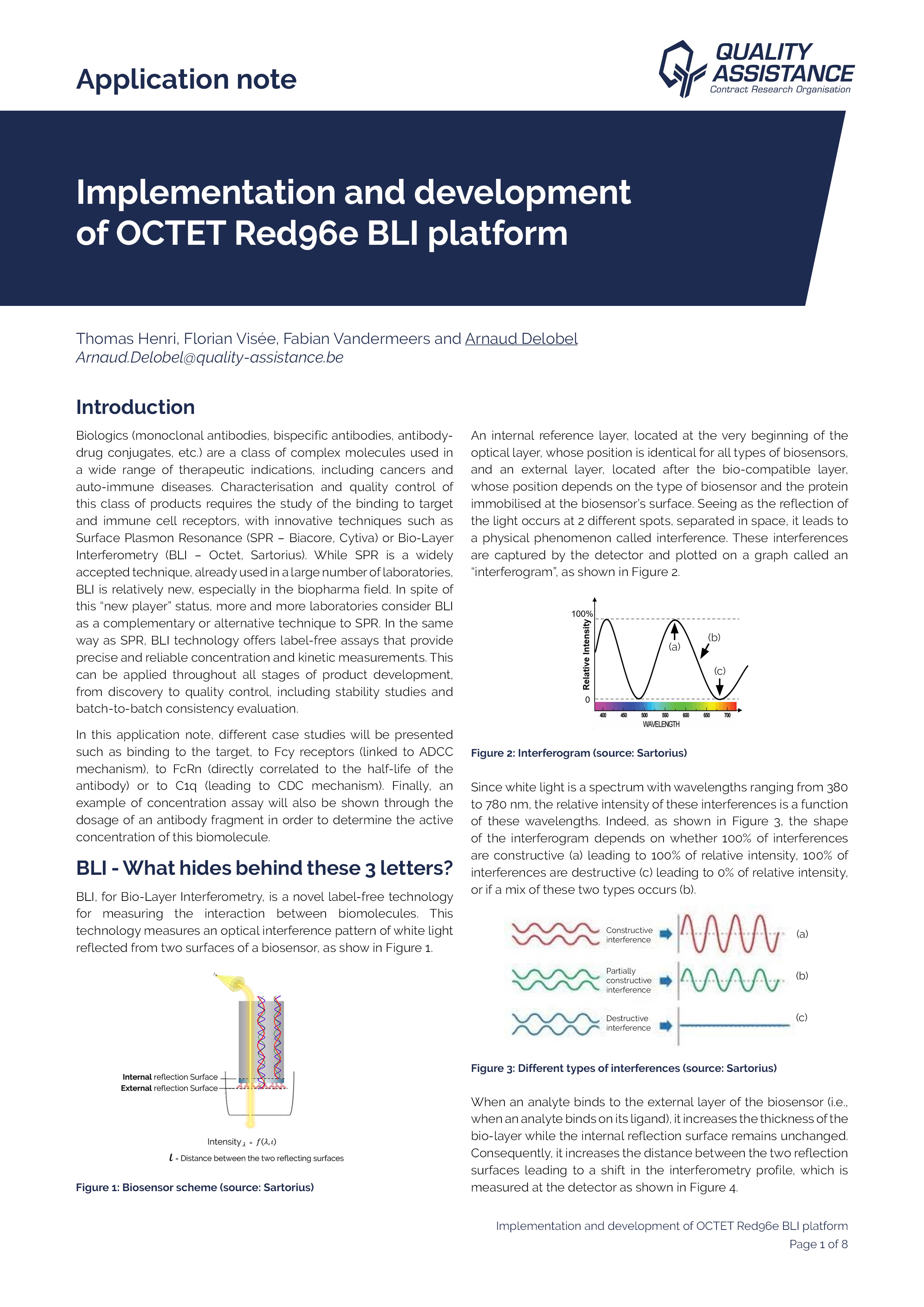
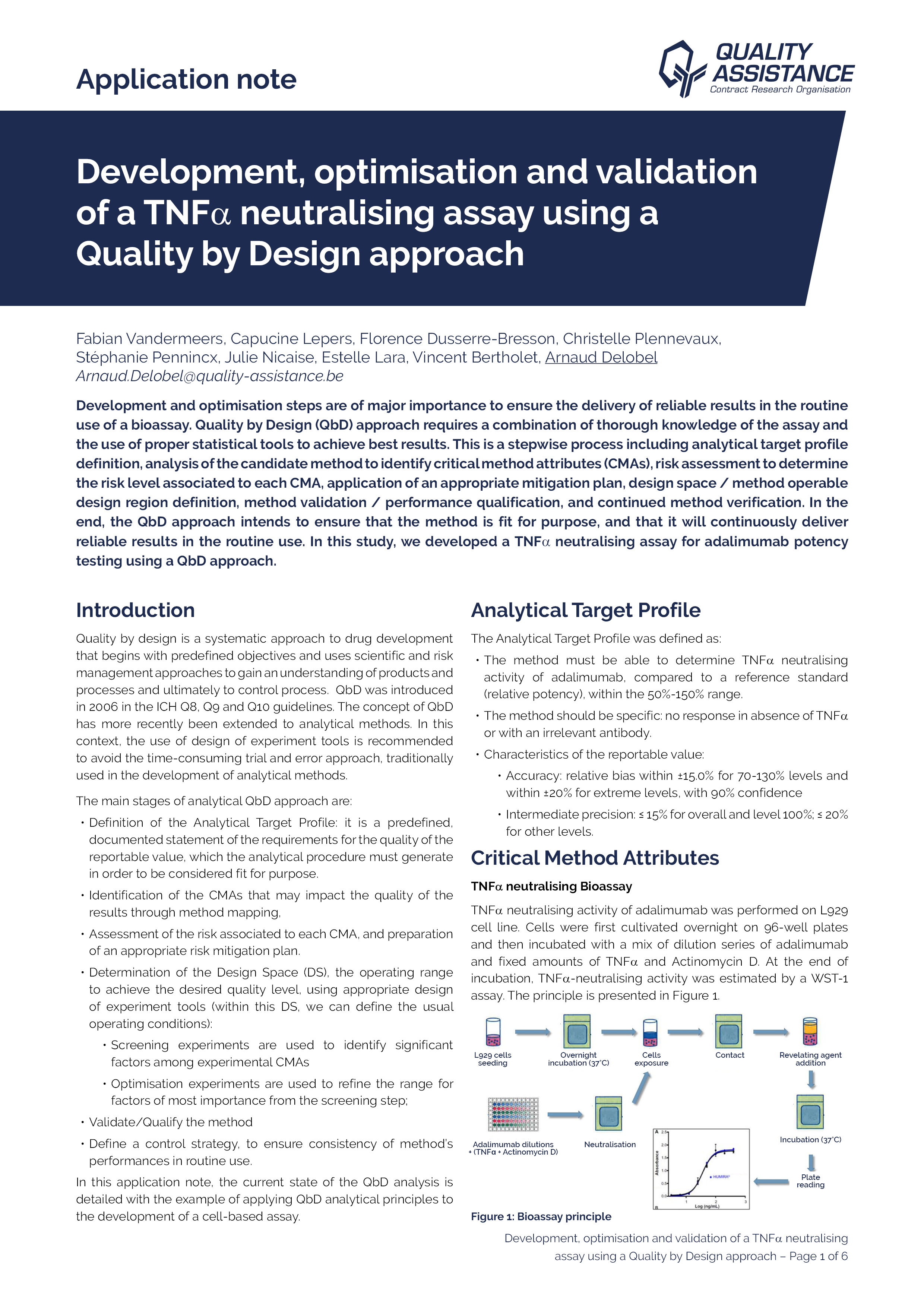
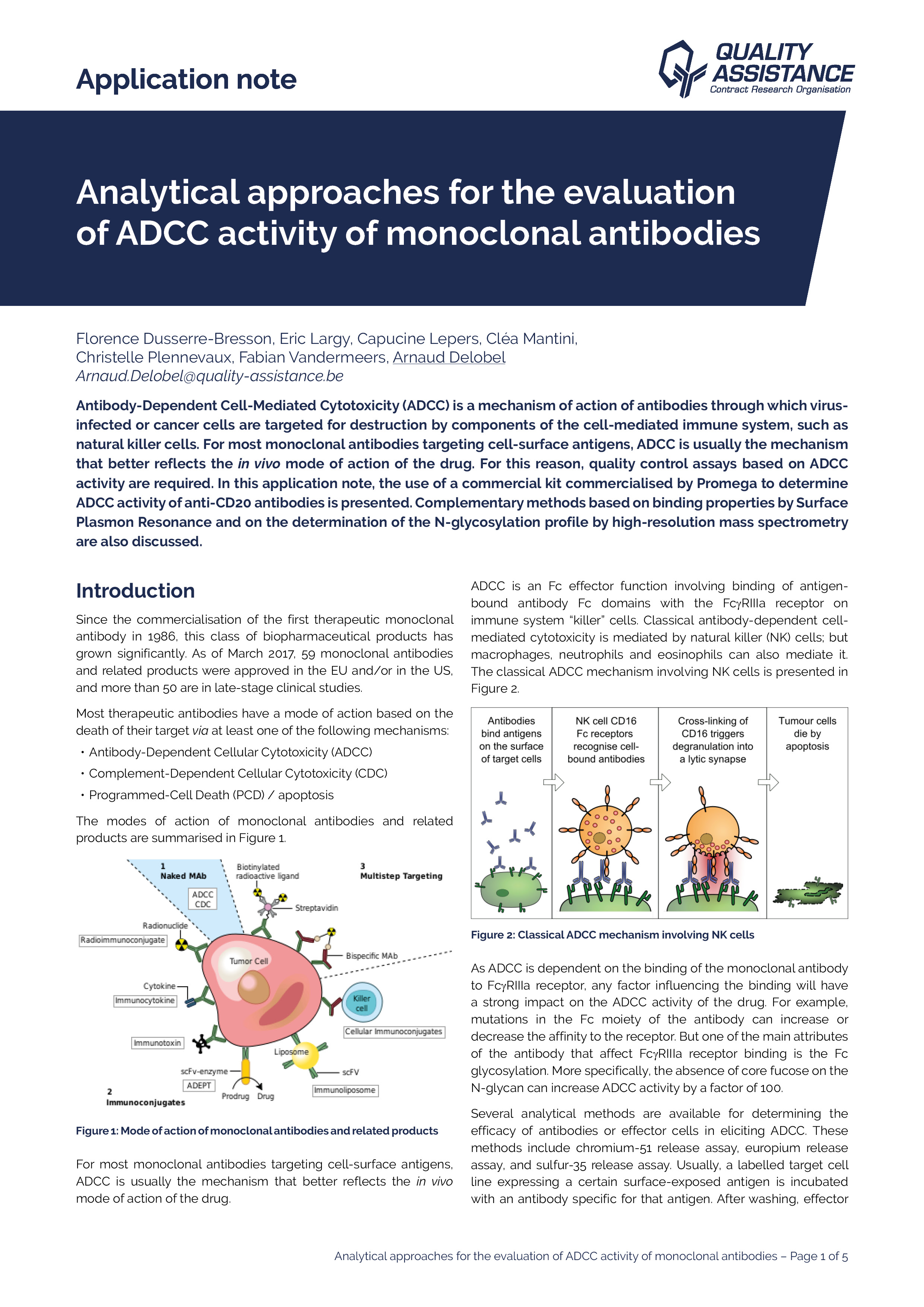
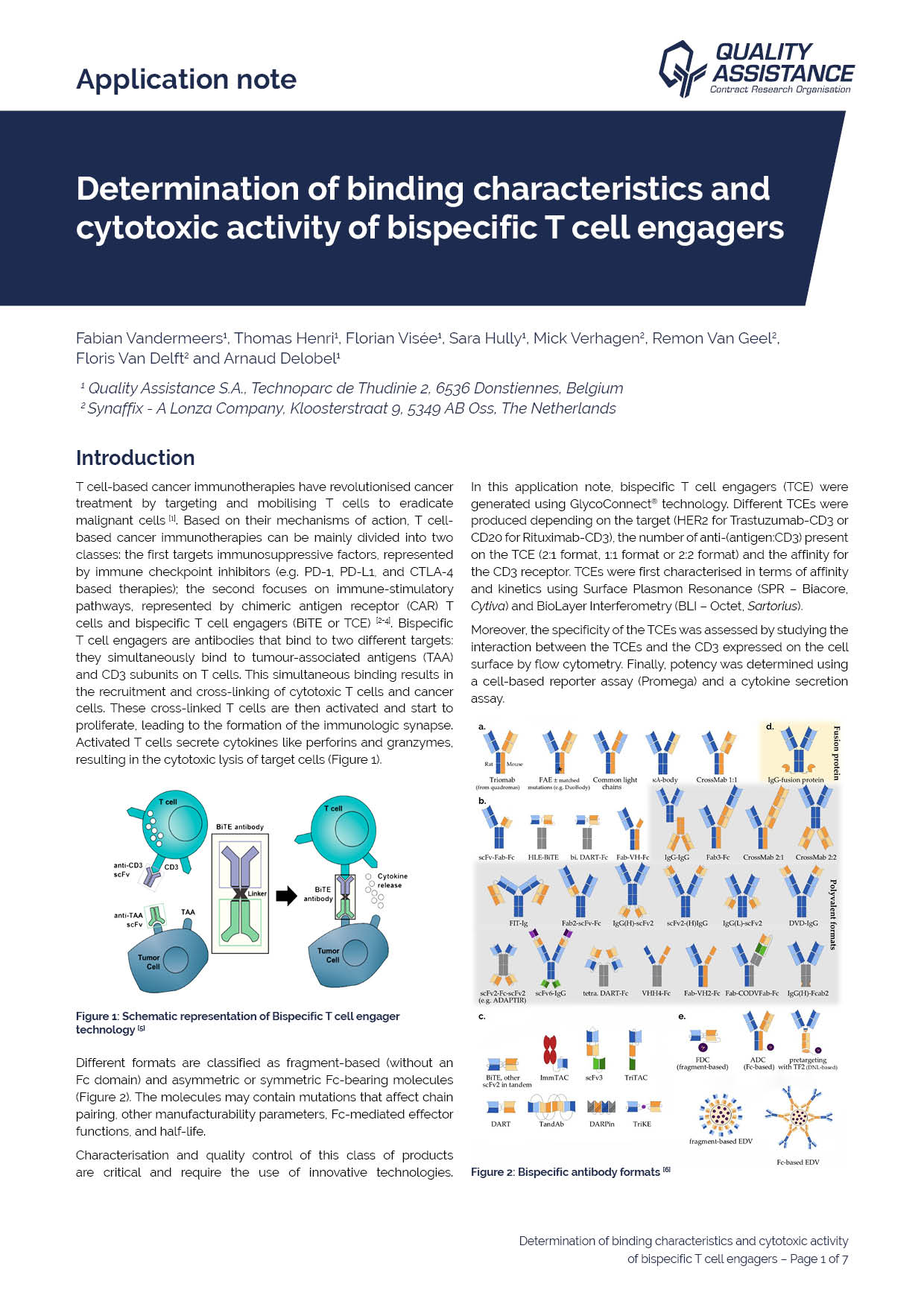
Biologics (monoclonal antibodies, bispecific antibodies, antibody-drug conjugates, etc.) are a class of complex molecules used in a wide range of therapeutic indications, including cancers and auto-immune diseases.


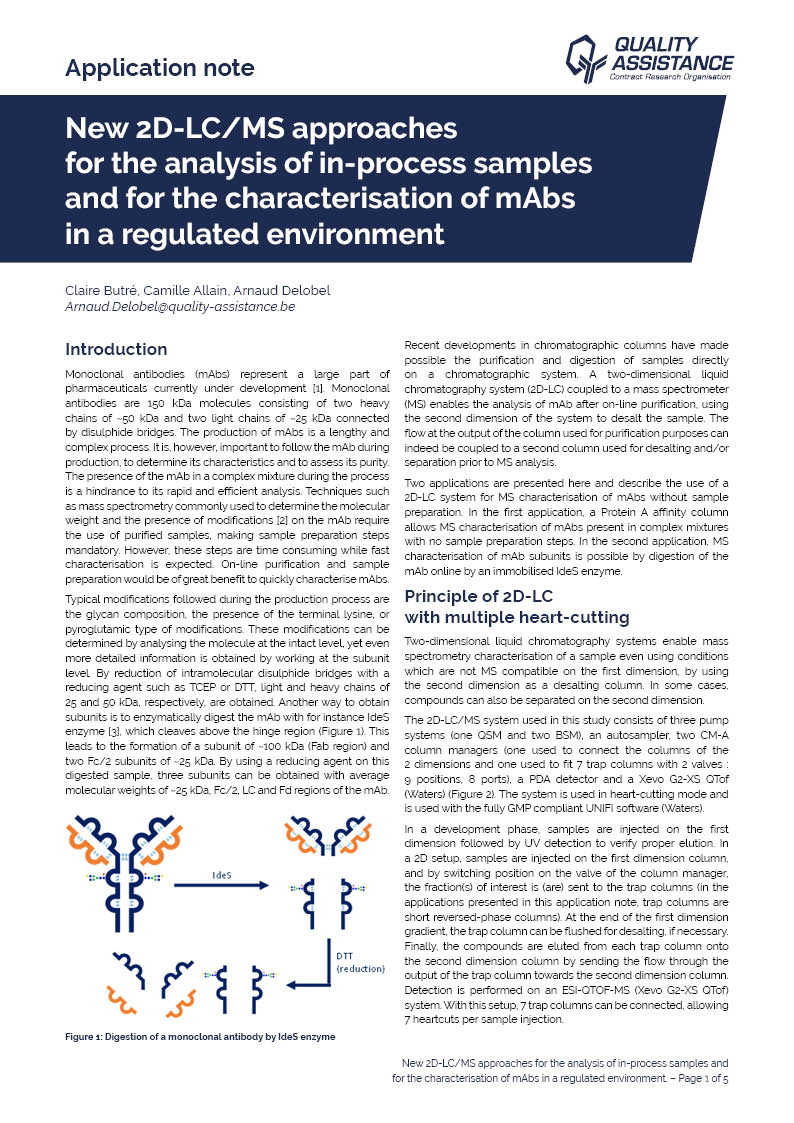
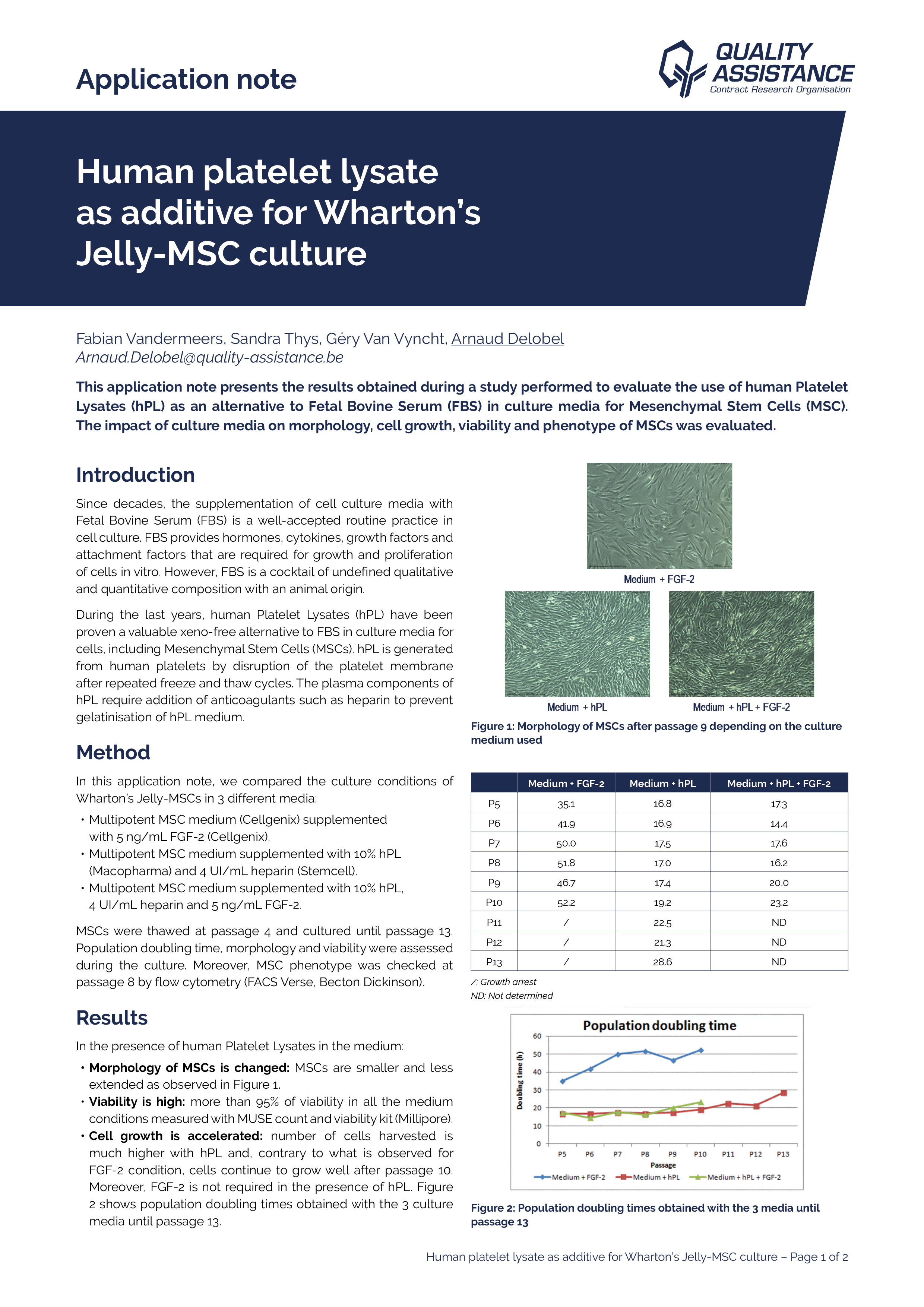
Biologics, and in particular monoclonal antibodies (mAbs), are an important class of therapeutics, and their market share keeps growing. The production of antibodies is a complex and lengthy process.
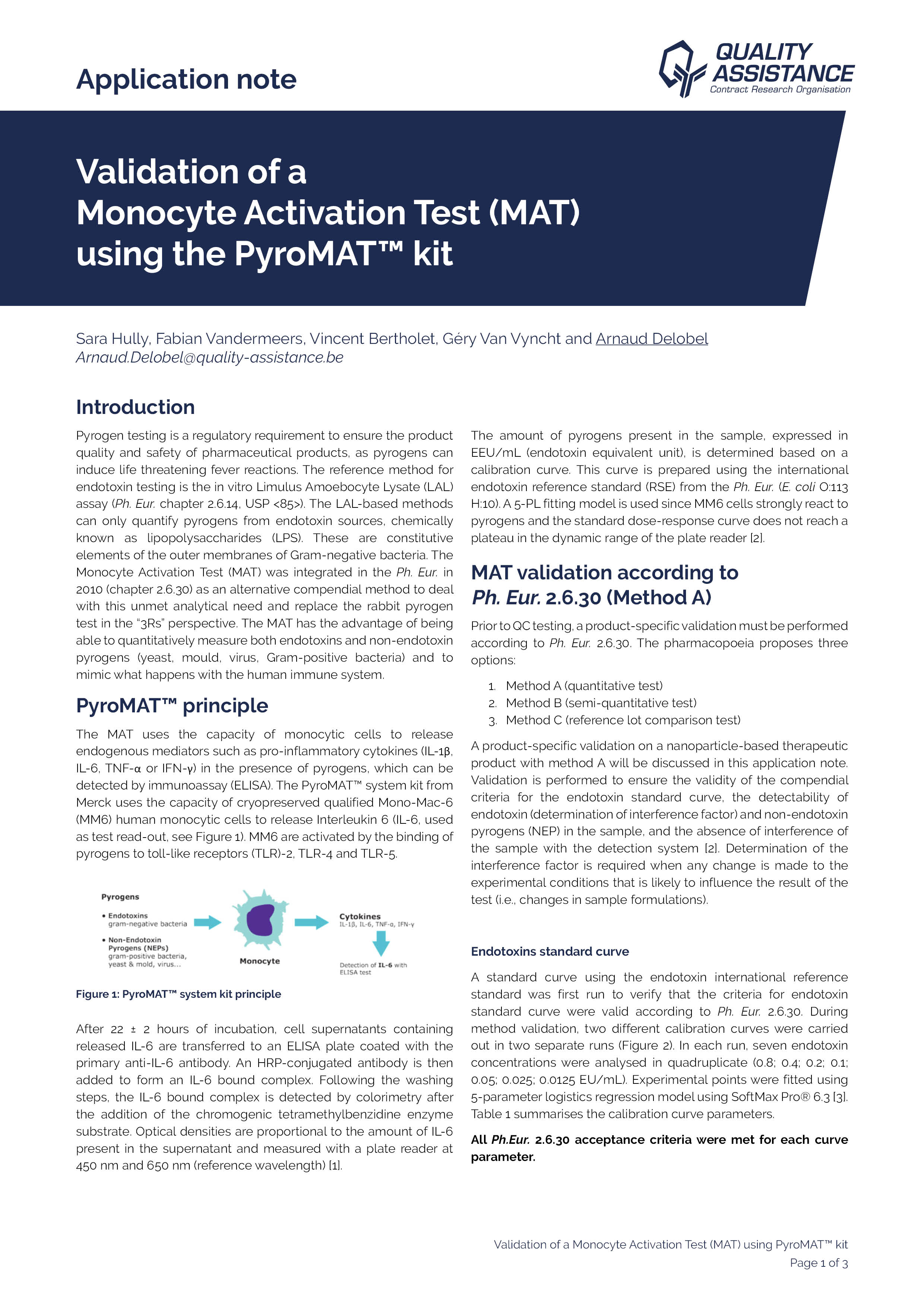
Pyrogen testing is a regulatory requirement to ensure the product quality and safety of pharmaceutical products, as pyrogens can induce life threatening fever reactions.

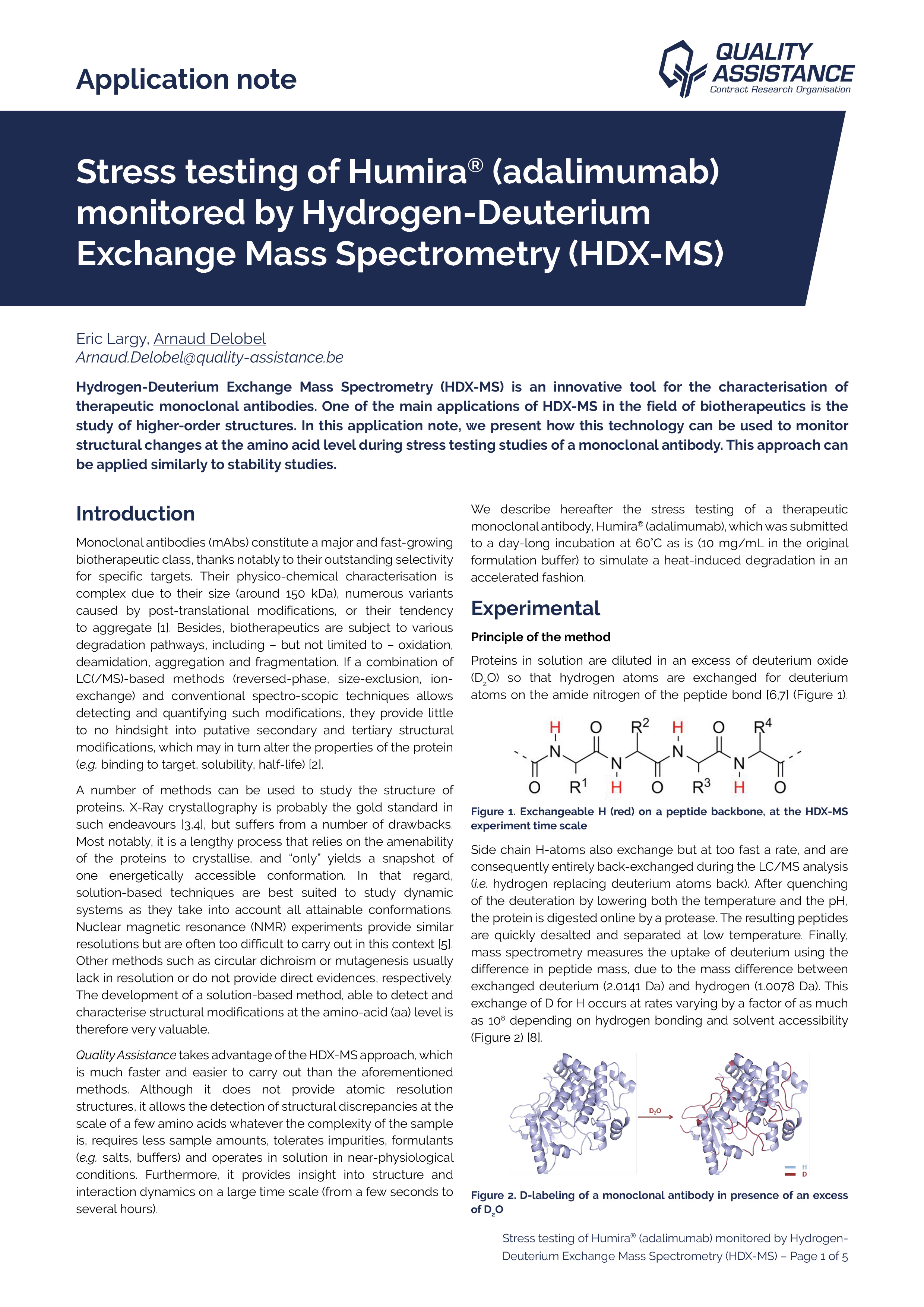
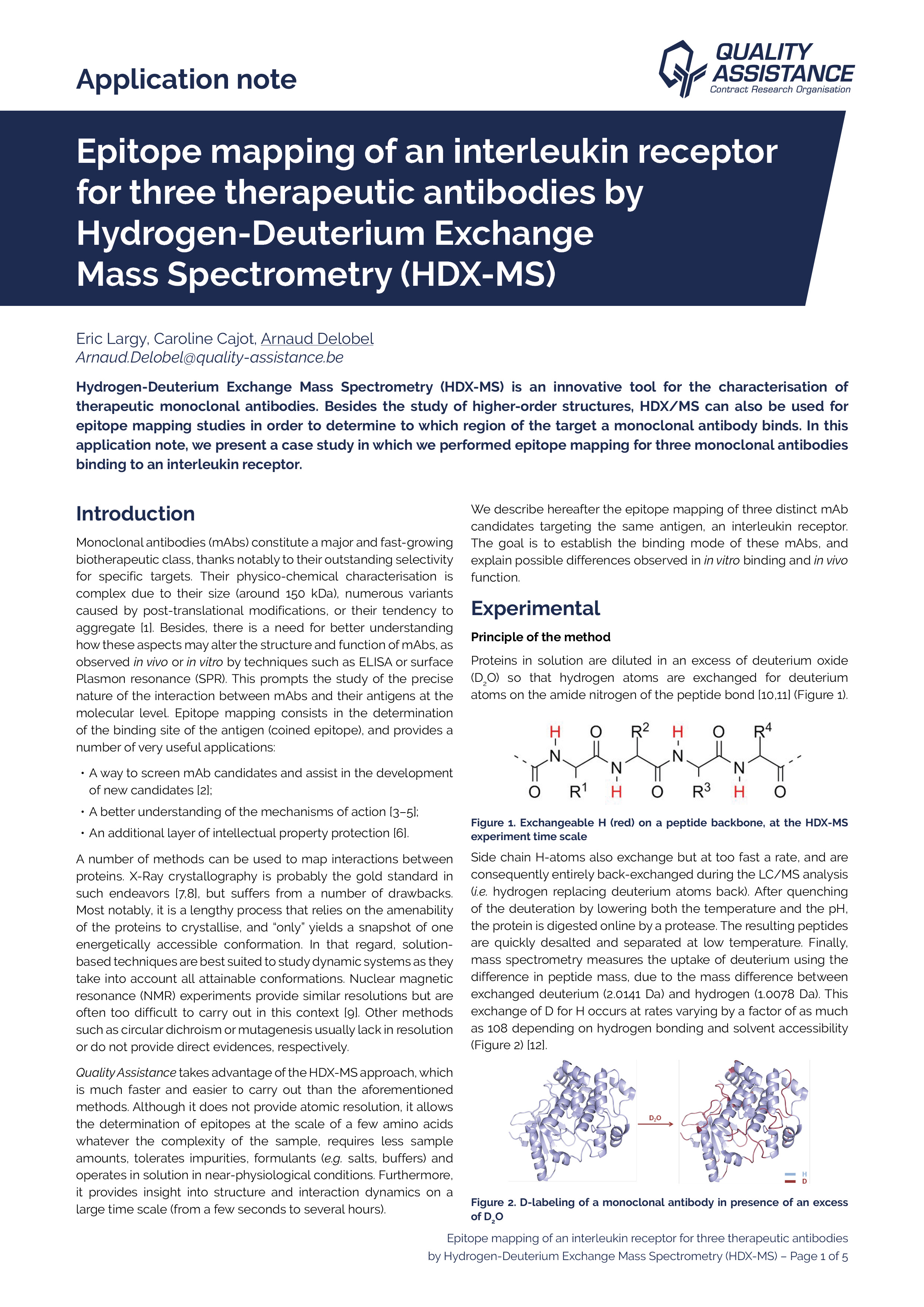
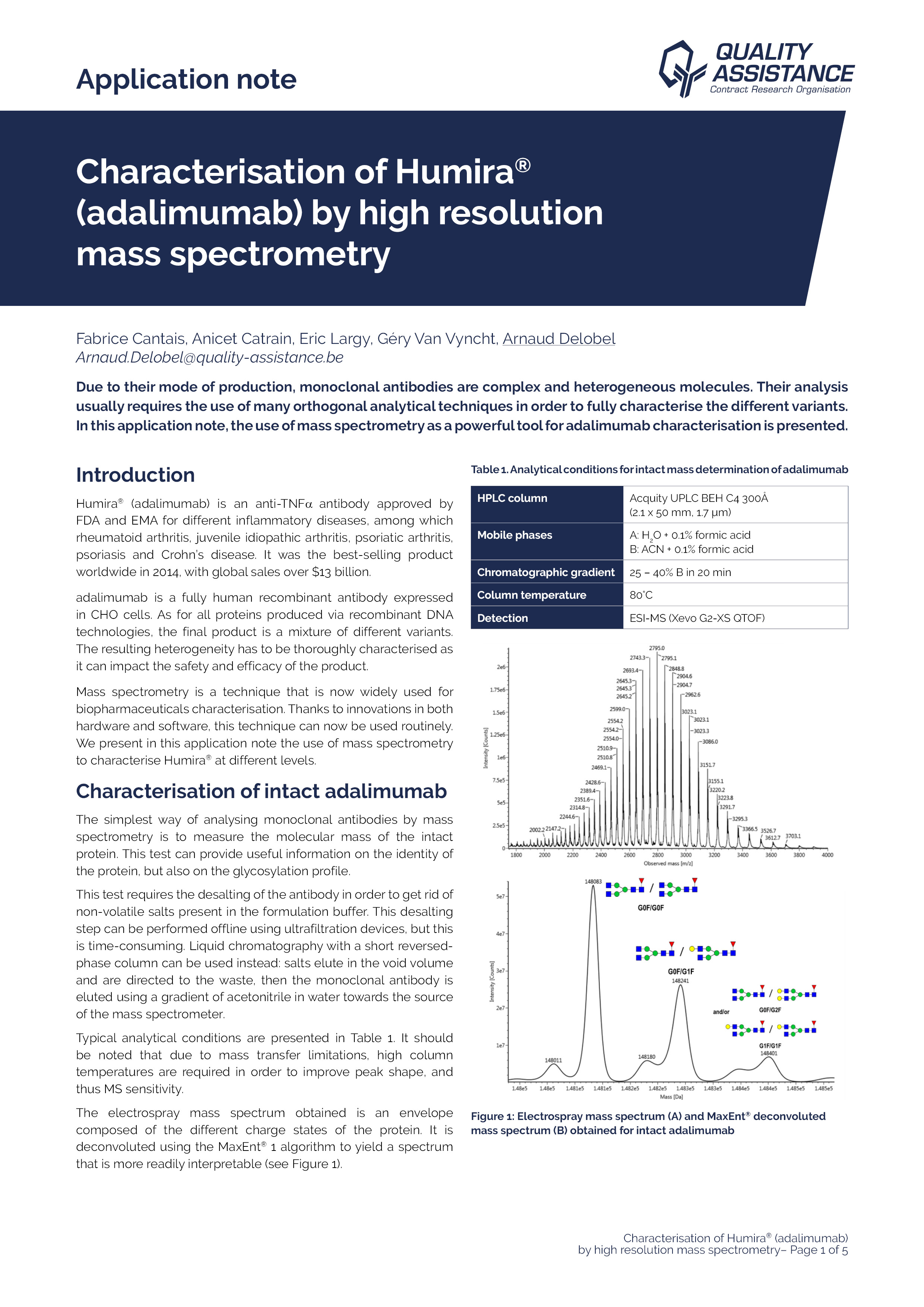
Monoclonal antibodies (mAbs) constitute a major and fast-growing biotherapeutic class, thanks notably to their outstanding selectivity for specific targets.

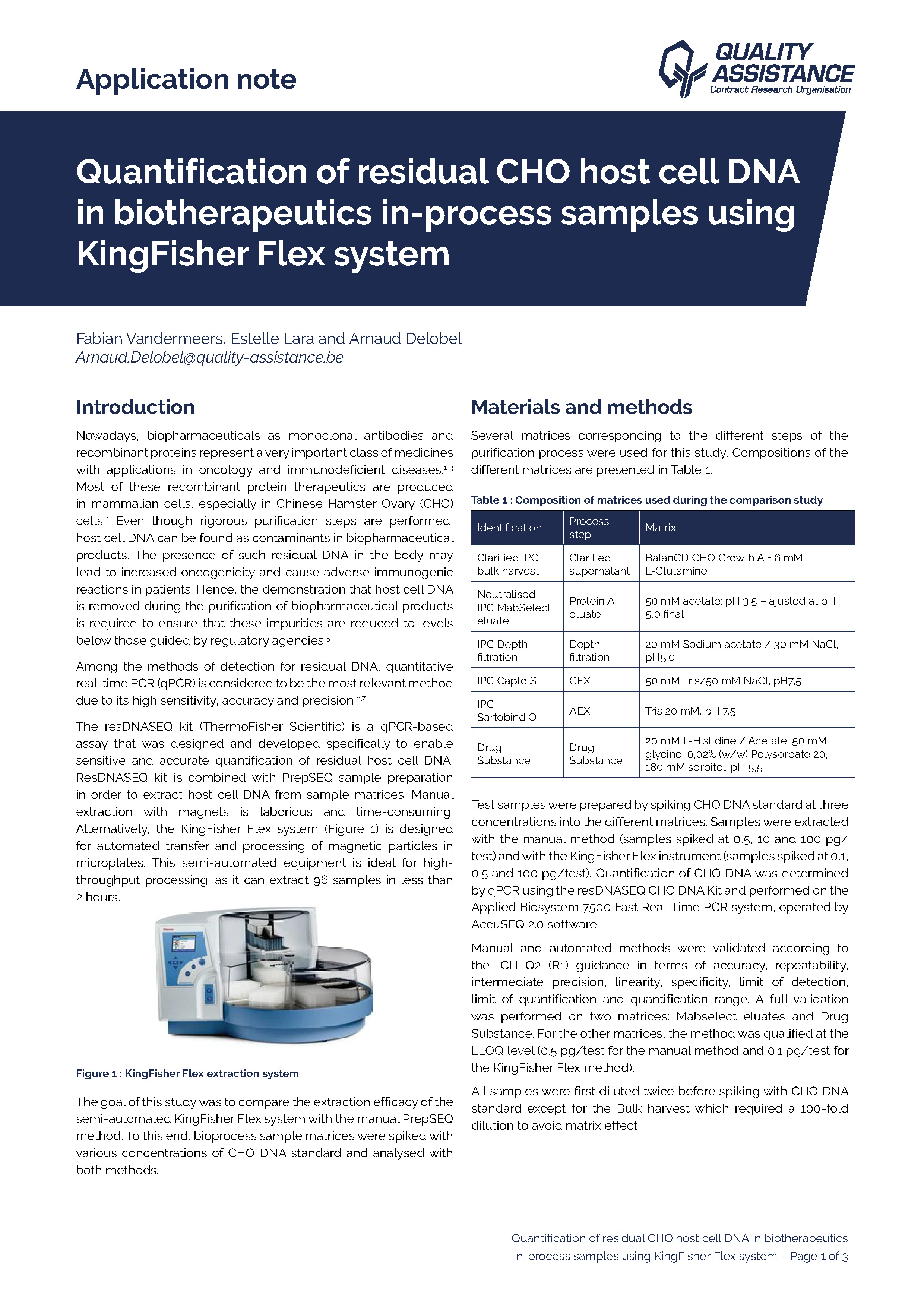
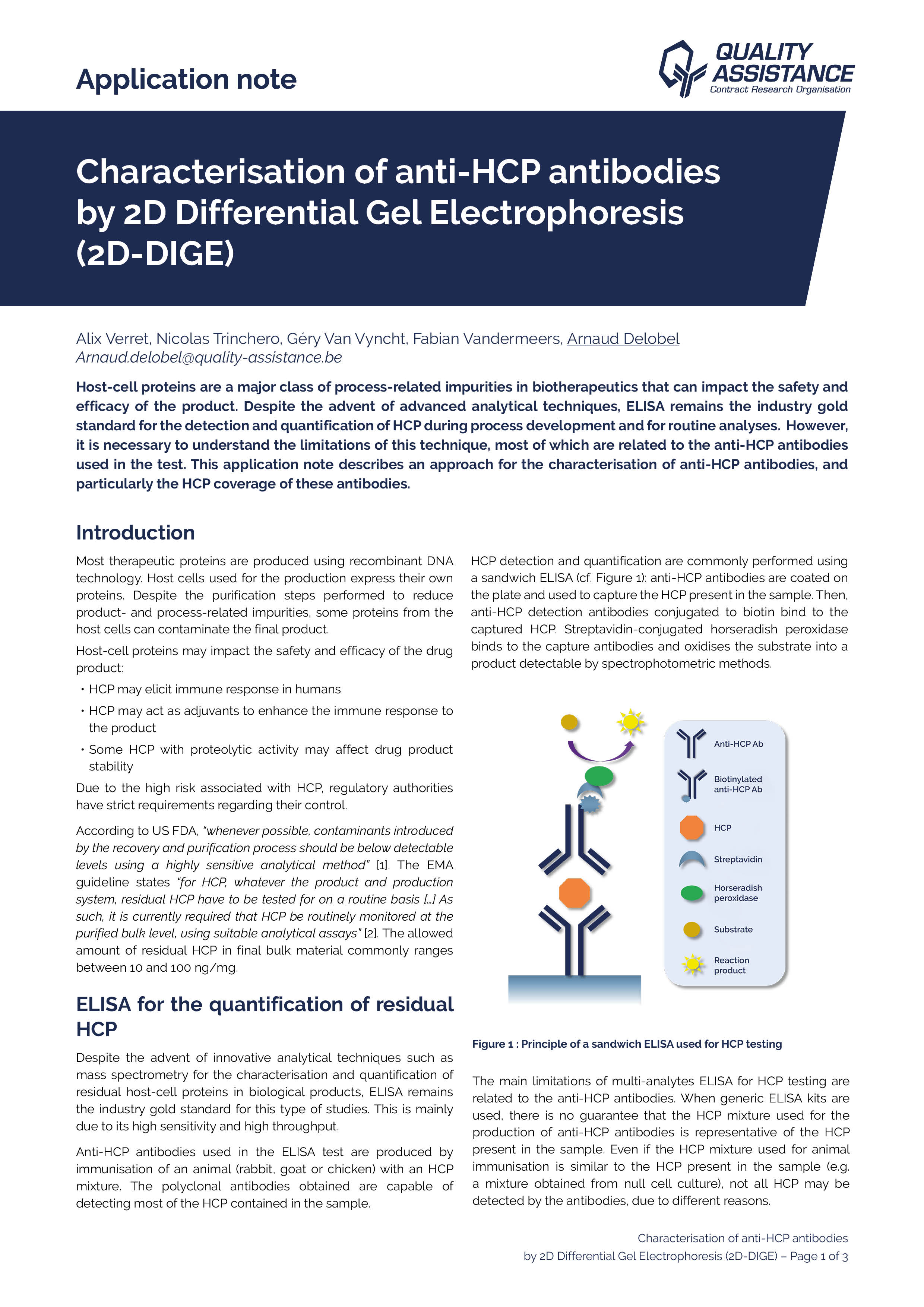
The presence of residual host cell DNA in biopharmaceutical products can induce severe side effects for patients.
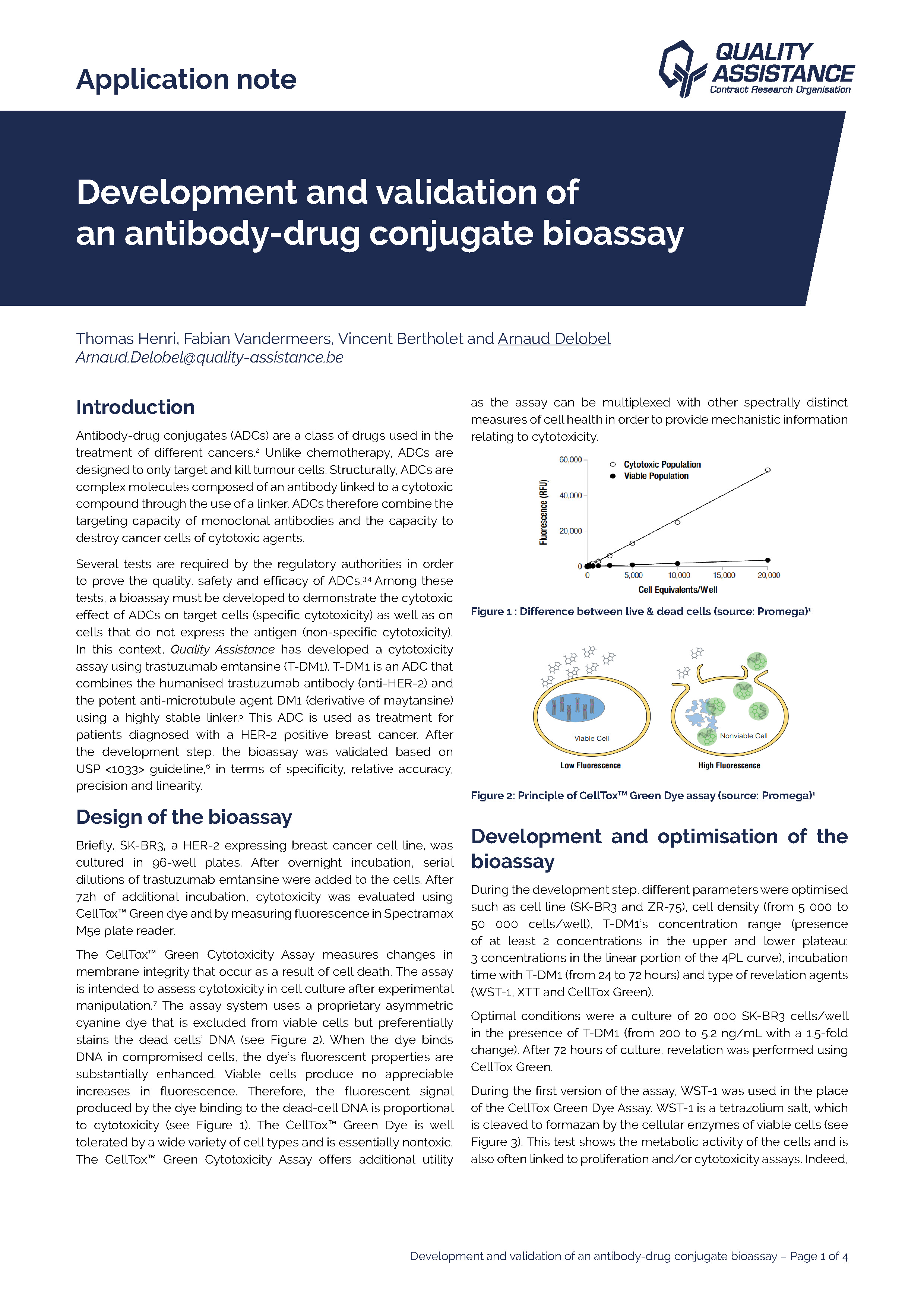
Antibody-drug conjugates (ADCs) are a class of drugs used in the treatment of different cancers. Unlike chemotherapy, ADCs are designed to only target and kill tumour cells. Structurally, ADCs are complex molecules composed of an antibody linked to a cytotoxic co
The development of liquid chromatography (LC) methods has long been done using a trial-and-error approach, also known as “one factor at a time” (OFAT). While this approach is the easiest to implement, it is both time-consuming and quite inefficient.
Traditional “absolute” methods of analysis for protein quantification include colorimetry, amino acid (AA) analysis and UV-Vis spectroscopy but each of these techniques has its limitations.
Cytokine profiling is a powerful tool to link the host immune system with disease pathogenesis and/or treatment efficacy.