Determination of binding characteristics and cytotoxic activity of bispecific T cell engagers
1 Quality Assistance S.A., Technoparc de Thudinie 2, 6536 Donstiennes, Belgium
2 Synaffix - A Lonza Company, Kloosterstraat 9, 5349 AB Oss, The Netherlands
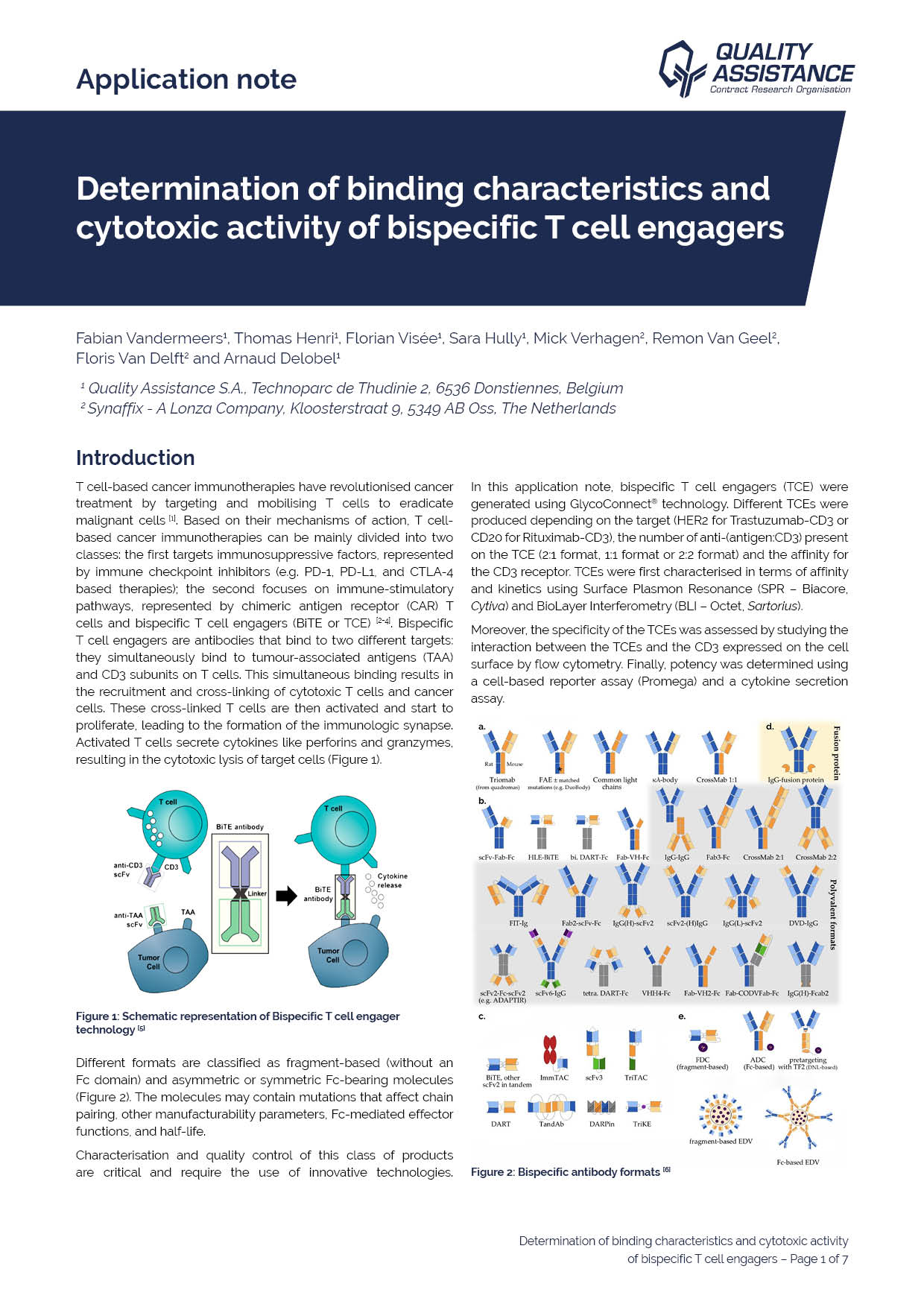
T cell-based cancer immunotherapies have revolutionised cancer treatment by targeting and mobilising T cells to eradicate malignant cells. Based on their mechanisms of action, T cell-based cancer immunotherapies can be mainly divided into two classes: the first targets immunosuppressive factors, represented by immune checkpoint inhibitors (e.g. PD-1, PD-L1, and CTLA-4 based therapies); the second focuses on immune-stimulatory pathways, represented by chimeric antigen receptor (CAR) T cells and bispecific T cell engagers (BiTE or TCE). Bispecific T cell engagers are antibodies that bind to two different targets: they simultanously bind to tumour-associated antigens (TAA) and CD3 subunits on T cells. This simultaneous binding results in the recruitment and cross-linking of cytotoxic T cells and cancer cells. These cross-linked T cells are then activated and start to proliferate, leading to the formation of the immunologic synapse. Activated T cells secrete cytokines like perforins and granzymes, resulting in the cytotoxic lysis of target cells.
Different formats are classified as fragment-based (without an Fc domain) and asymmetric or symmetric Fc-bearing molecules. The molecules may contain mutations that affect chain pairing, other manufacturability parameters, Fc-mediated effector
functions, and half-life.
Characterisation and quality control of this class of products are critical and require the use of innovative technologies. In this application note, bispecific T cell engagers (TCE) were generated using GlycoConnect® technology. Different TCEs were produced depending on the target (HER2 for Trastuzumab-CD3 or CD20 for Rituximab-CD3), the number of anti-(antigen:CD3) present on the TCE (2:1 format, 1:1 format or 2:2 format) and the affinity for the CD3 receptor. TCEs were first characterised in terms of affinity and kinetics using Surface Plasmon Resonance (SPR – Biacore, Cytiva) and BioLayer Interferometry (BLI – Octet, Sartorius).
Moreover, the specificity of the TCEs was assessed by studying the interaction between the TCEs and the CD3 expressed on the cell surface by flow cytometry. Finally, potency was determined using a cell-based reporter assay (Promega) and a cytokine secretion assay.