In-depth characterisation of antibody charge variants by icIEF fractionation coupled to intact mass spectrometry and peptide mapping
Imaged capillary isoelectric focusing (icIEF) is the gold standard technique for the determination and relative quantification of biotherapeutics charge variants, considered as a critical quality attributes (CQA).
Capillary isoelectric focusing is a high-resolution electrophoretic separation technique that allows for the precise determination of the isoelectric point (pI) of recombinant proteins, such as antibodies and other biomolecules. The different pI values observed reflect charge heterogeneity, post-translational modifications and isoforms in the analysed sample.
However, this technique does not provide any information regarding the type of modification leading to the pI shift and requires deeper analytical capabilities. Additional tools like coupling a capillary electrophoresis system to a mass spectrometer may be used. Orthogonal techniques, such as SCX-MS and 2D-LC-MS can also be set up but would present different profiles than icIEF, hardening the correlation between the different peaks observed and isoforms / variants identification.
Recently, Bio-Techne released the new MauriceFlex™ instrument capable of performing fractionation after isoelectric focusing of a sample. Fractions obtained are free of MS-incompatible reagents
(such as methyl cellulose) and thus can be used to perform charge variants characterisation.
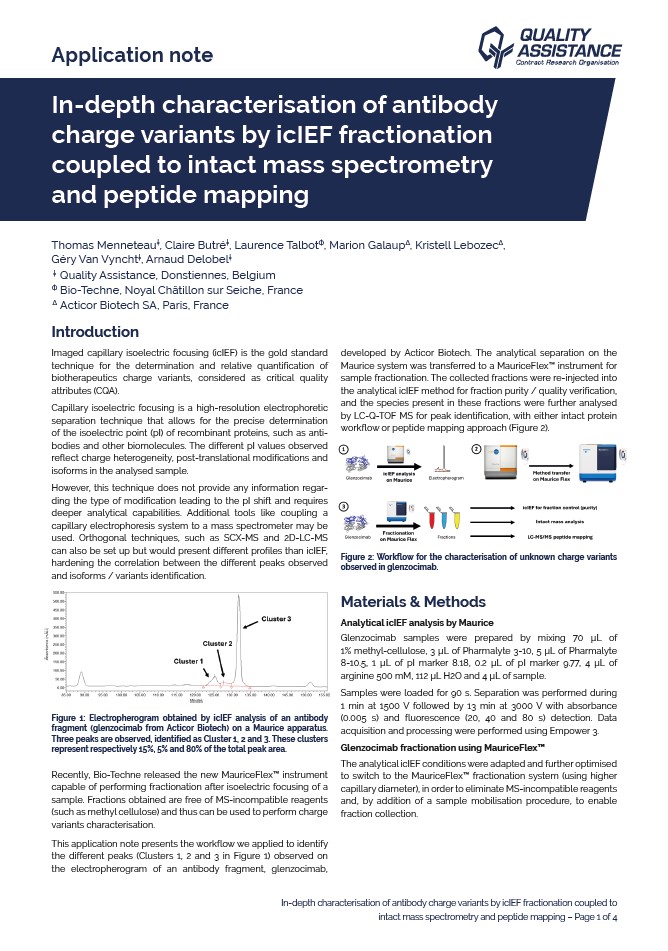
This application note presents the workflow we applied to identify the different peaks (Clusters 1, 2 and 3 in Figure 1) observed on the electropherogram of an antibody fragment, glenzocimab, developed by Acticor Biotech. The analytical separation on the Maurice system was transferred to a MauriceFlex™ instrument for sample fractionation. The collected fractions were re-injected into the analytical icIEF method for fraction purity / quality verification, and the species present in these fractions were further analysed by LC-Q-TOF MS for peak identification, with either intact protein workflow or peptide mapping approach.
The combination of MauriceFlex™-assisted icIEF fractionation with mass spectrometry led to the identification of three modifications of glenzocimab, a N-term pyroglutamic acid, a glycation and a deamidation, corresponding respectively to the Cluster 3, 1 and 2 initially identified in icIEF analysis. This type of approach can be applied to any purified protein to identify charge variants when peak separation is sufficient to collect the species in different fractions. Indeed, one major advantage of this analysis is that similar profiles and elution order as in classic analytical icIEF experiments were observed. However, even if method transposition from Maurice to MauriceFlex™ appeared quite straightforward, icIEF fractionation requires optimisation and confirmation to match the different investigated peaks to the collected fractions. The type of mass spectrometry analysis performed for the identification of post-translational modifications should also be adjusted depending on their nature: indeed, observation of deamidation by intact mass is not straightforward, as its molecular weight can be considered as mass error due to the size of intact antibodies. In case of no prior information regarding the nature of the modifications, both intact and peptide mapping approaches should be performed.