
Advanced characterisation of therapeutic mRNA with HRMS
28th January 2025
Advanced characterisation of therapeutic mRNA with HRMS
Achieve unparalleled precision in evaluating Critical Quality Attributes for your therapeutic mRNA with advanced LC-MS solutions
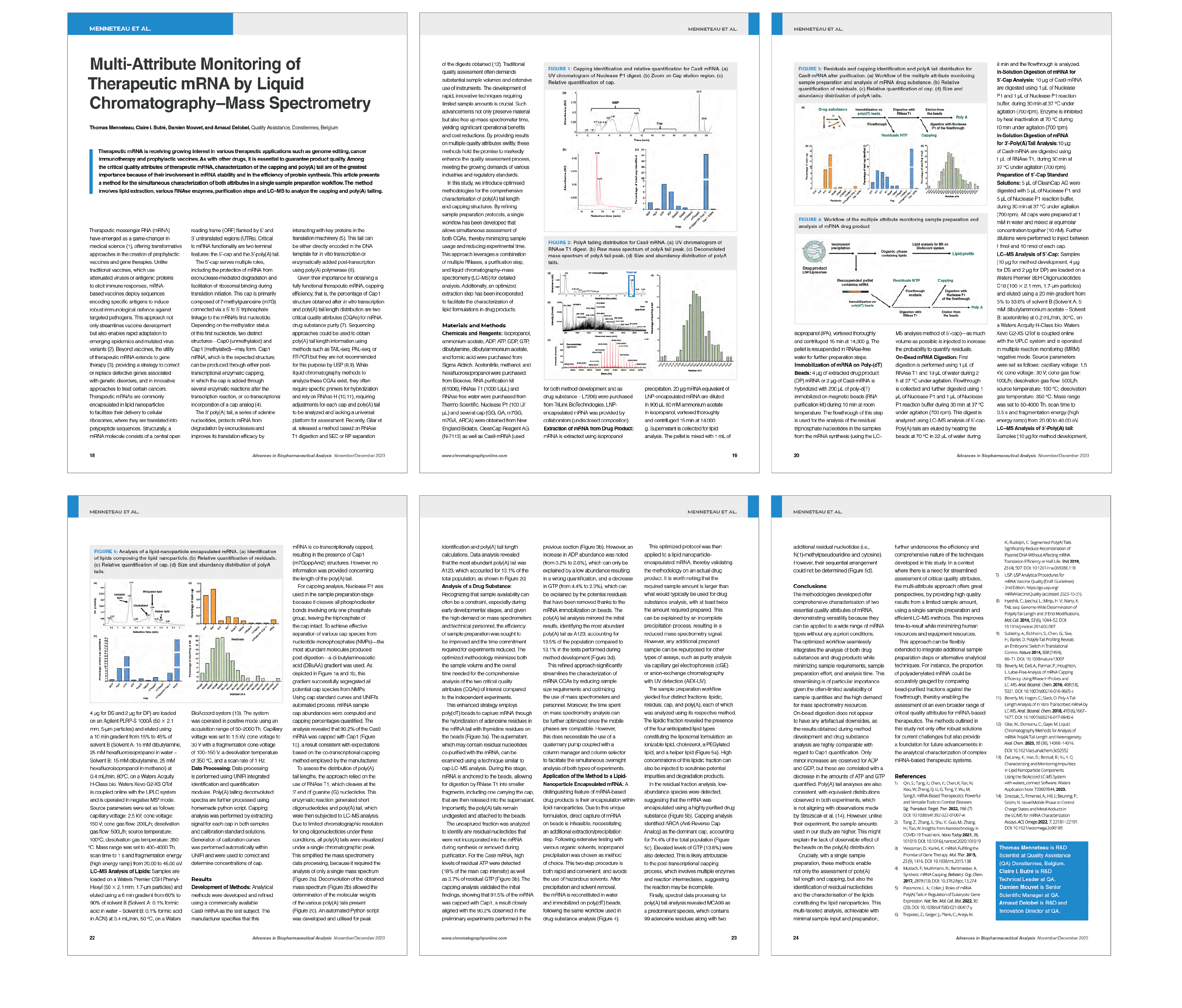
Therapeutic mRNA is revolutionising modern medicine, driving breakthroughs in vaccines and personalised cancer therapies. To ensure the stability, efficacy, and safety of these innovative treatments, a precise characterisation of Critical Quality Attributes (CQAs) such as sequence confirmation, 5' cap structure and poly(A) tail length is essential.
At Quality Assistance, we use cutting-edge HRMS (High-Resolution Mass Spectrometry) platforms to deliver comprehensive and simultaneous analyses, streamlining your development processes while conserving valuable resources.
Key benefits of our solution
Multi-attribute analysis of mRNA drug substances and drug products
Our integrated LC-MS workflow delivers:
- Identification and accurate quantification of 5' cap, critical for mRNA stability and translational efficiency.
- Precise measurement of poly(A) tail length, essential for regulating mRNA half-life and protein synthesis.
- Significant reductions in sample requirements and analysis time, making it ideal for early-stage development where material is often limited.
- Possibility to analyse simultaneously residual NTPs and lipids (for mRNA-LNP drug products)
Case Study: our workflow, featured in LCGC Europe, combines comprehensive characterisation of these critical attributes in a single, streamlined process. This approach accelerates development while ensuring reproducible, high-quality results.
Therapeutic mRNA sequencing
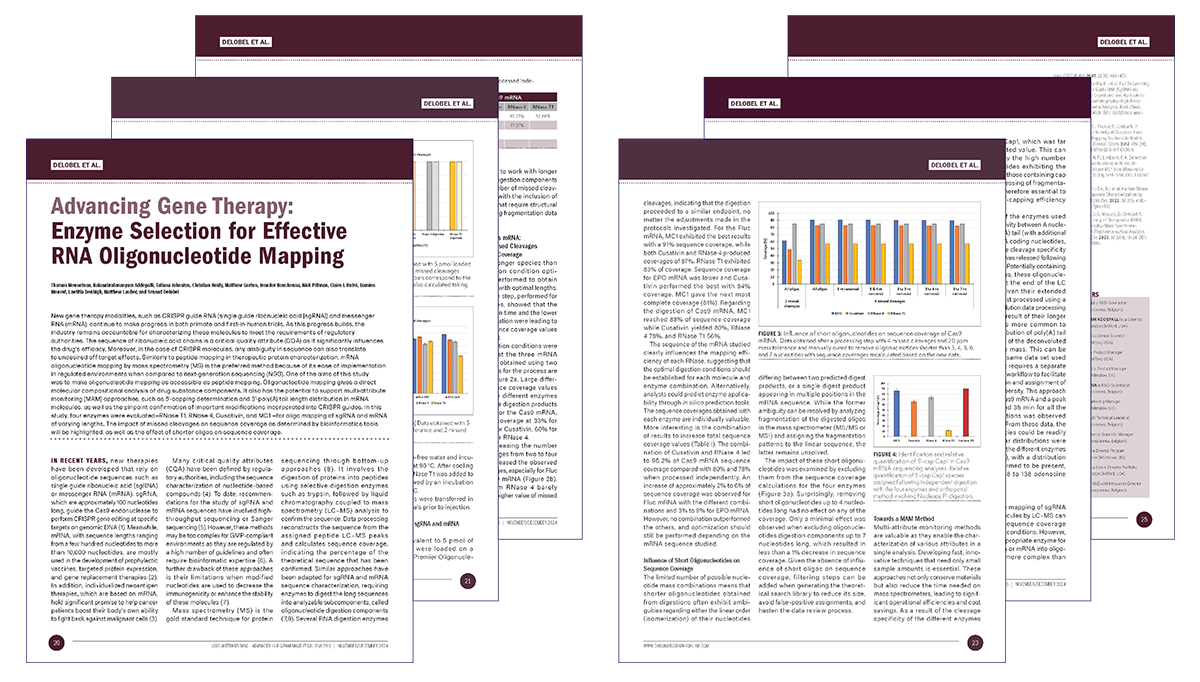
We developed, in collaboration with Waters Corporation, an innovative approach using new enzymes and advanced bioinformatics workflows for therapeutic mRNA sequencing, based on oligonucleotide mapping methodology, providing high-sequence coverage for efficient identification testing.
Webinar: Gain in-depth insights into our advanced mRNA sequencing techniques by watching the replay of the webinar co-hosted with Waters Corporation: "Cracking the Code: analysing mRNA and sgRNA with advanced enzymes, mass spectrometry and informatics"
 |  |
Why Partner with Quality Assistance for mRNA?
✅ Extensive expertise in the characterisation of complex therapeutic modalities.
✅ Access to state-of-the-art HRMS technologies delivering high-precision and reproducible results
✅ Tailored analytical workflows addressing the specific challenges of mRNA therapeutic development.