World ADC 2025
London, UK
Quality Assistance will attend World ADC 2025
Curious to know how Quality Assistance can help you boost the development of your Antibody-Drug Conjugates?
Meet our Charlotte Lentz, Louis Lefebvre and Géry Van Vyncht at booth 21 during the conference!

About the event
Visit https://worldadc-europe.com/ for more information
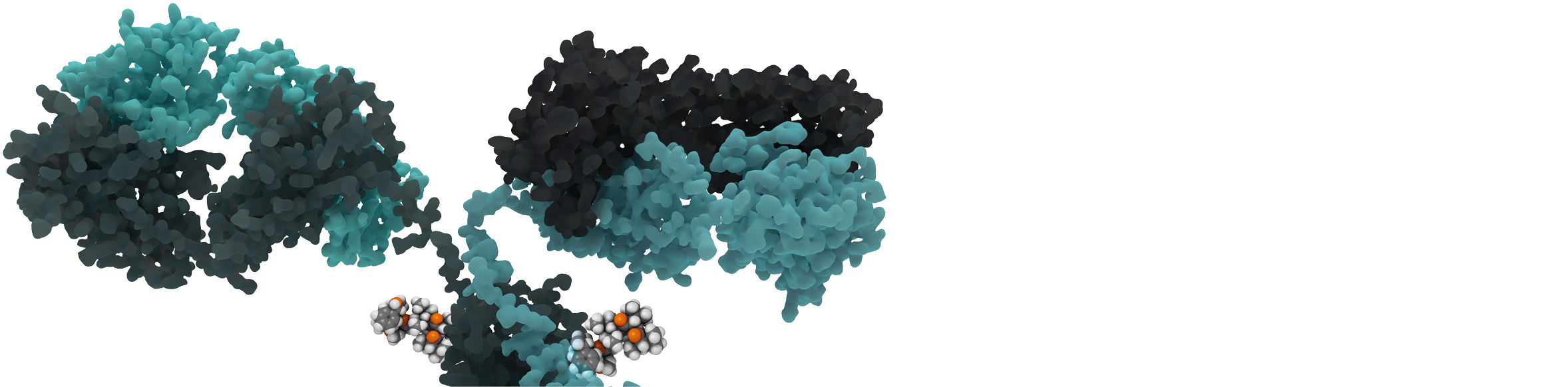
YOUR ANALYTICAL PARTNER
With a full analytical package for Biotherapeutics, Quality Assistance provides scientific and technical support to biopharmaceutical companies developing Antibody-Drug Conjugates.
Thanks to our combined experience with small molecule cytotoxics and monoclonal antibodies, we can provide full analytical support for your projects. We offer a wide range of services for all payload categories (maytansine derivatives, auristatins, etc.) and all conjugation strategies (lysine, cysteine, site-specific).
Throughout non-clinical and clinical development, our scientific team provides you with customised solutions in terms of analytical protocols and innovative technologies to help you move your product towards registration.

QUALITY ASSISTANCE IS THE ONE-STOP SHOP FOR ANALYTICAL SERVICES
We provide:
- customised solutions in terms of analytical protocols and innovative technologies throughout non-clinical and clinical development.
- a full range of equipment in Chromatography, Mass Spectrometry, Biochemistry, Cell Biology, Pharmaceutical Technologies and Microbiology, to meet all the challenges of developing and validating methods.
- regulatory, scientific and technical excellence with a problem-solving approach
- compliance with all applicable EMA, FDA and ICH regulations
- GMP, GLP, GCLP/GCP environment
Outsource your analytical needs on one site for more efficiency including:
- Analytical development
- Validation of analytical methods as per ICH guidelines
- Characterisation of ADCs at intact, sub-unit and peptide levels
- Bioassays
- Bioanalytical services
- Stability studies
- Batch testing