TIDES Europe 2024
Hamburg, Germany
Join Quality Assistance at TIDES Europe 2024
The Oligonucleotide & Peptide Therapeutics Event
MEET OUR TEAM THERE
Jordan Cossu and Julie Jia, our Business Development representatives, and Damien Mouvet, Scientific Director Senior, will be delighted to meet you at booth #512. Schedule a meeting at our stand to learn how Quality Assistance can help you with the registration process of your oligonucleotides, peptides and mRNA-based therapeutics
We are experts in...
OLIGONUCLEOTIDES
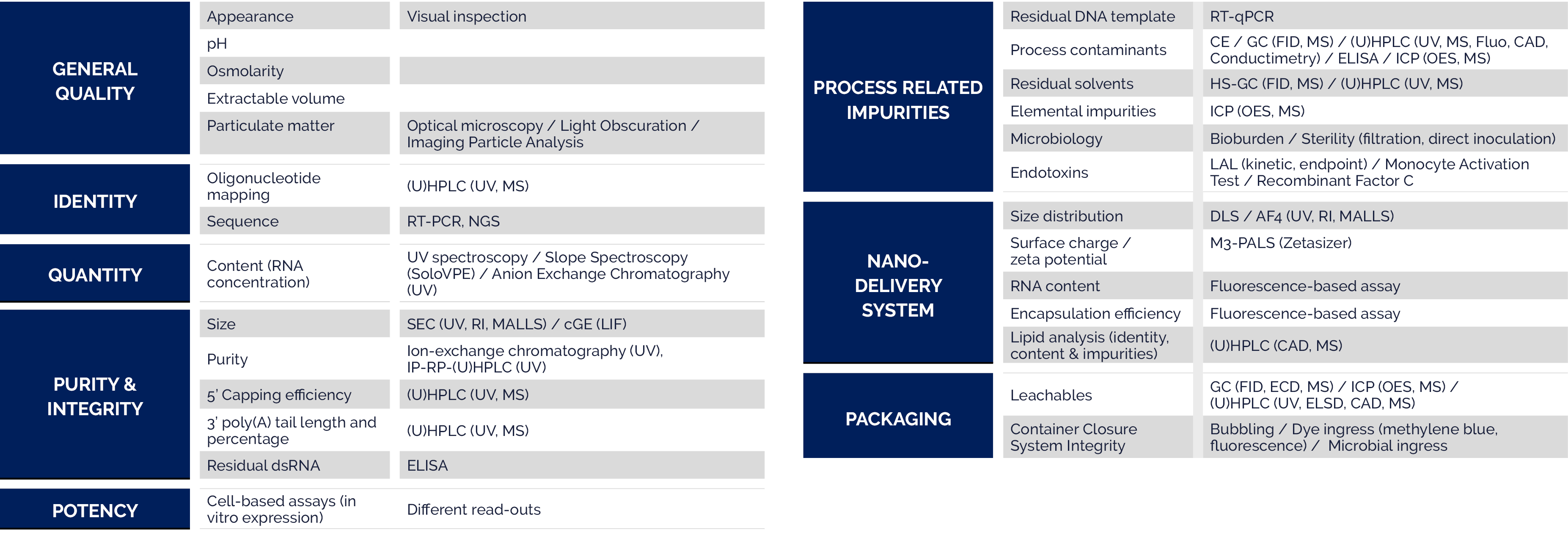
Due to their relatively large size as compared to typical small molecule drugs, there are many technical challenges associated with the analysis of oligonucleotides. With a highly experienced staff and state-of-the-art premises and equipment, Quality Assistance is your partner of choice to overcome your analytical hurdles and facilitate the registration process.

PEPTIDES
Therapeutic peptides are at the crossroads of small molecules and proteins, requiring specific analytical packages depending on whether they are of recombinant or synthetic origin. Quality Assistance provides customised solutions in terms of analytical protocols and innovative technologies to help you move your peptide through non-clinical and clinical development towards registration.

mRNA
mRNA is a new class of therapeutics designed to instruct patients’ own cells to produce a protein that can treat, cure or prevent diseases. mRNA therapeutics are being developed as vaccines against infectious diseases, as cancer immunotherapy, and as protein replacement therapy for rare diseases.
Building on its considerable experience in the analysis of complex innovative drugs, Quality Assistance is continuously advancing ways to support the development of your mRNA-based therapeutics and expanding its related machinery and equipment.
Click here for more information about the conference.
Schedule a meeting with our team: