ESGCT Congress 2024
Rome, Italy
Meet Quality Assistance at the European Society of Gene & Cell Therapy Congress
The ESGCT Annual Congress is a significant event in the field of gene and cell therapy. It brings together scientists, clinicians, regulatory authorities, and industry leaders to discuss the latest advancements, research findings and innovations in gene and cell therapy.
MEET US THERE
Join this exciting event and meet Louis Lefebvre and Rémy Delmotte, Key Account Managers, on booth A22. Damien Mouvet and Pedro Alves, Scientific Managers, will also attend the congress. Make sure to exchange with them on the latest Gene and Cell Therapy trends and discuss your analytical needs.

We are experts in…
CELL-BASED MEDICINAL PRODUCTS
Cell-Based Medicinal Products are complex and require state-of-the-art analytical methodologies to evaluate their quality, safety and efficacy. Quality Assistance provides you with the scientific and technical support to develop and/or optimise these methods for use in a GxP-regulated environment.
Throughout non-clinical and clinical development, our experts provide you with the customised solutions in terms of analytical protocols and innovative technologies to help you move your product from discovery to the market place.

VIRAL VECTORS
Due to their complex structure and size, as well as a constantly evolving regulatory landscape, the physico-chemical and biological characterisation of viral vector-based products is challenging.
With extensive experience in the analysis of innovative products and thanks to a continuous investment in new technologies and machinery, Quality Assistance can assist you in the development of your recombinant Adeno-Associated Viral Vectors (rAAVs) products from early phases to commercialisation.
mRNA
mRNA is a new class of therapeutics designed to instruct patients’ own cells to produce a protein that can treat, cure or prevent diseases. mRNA therapeutics are being developed as vaccines against infectious diseases, as cancer immunotherapy, and as protein replacement therapy for rare diseases.
Building on its considerable experience in the analysis of complex innovative drugs, Quality Assistance is continuously advancing ways to support the development of your mRNA-based therapeutics and expanding its related machinery and equipment.
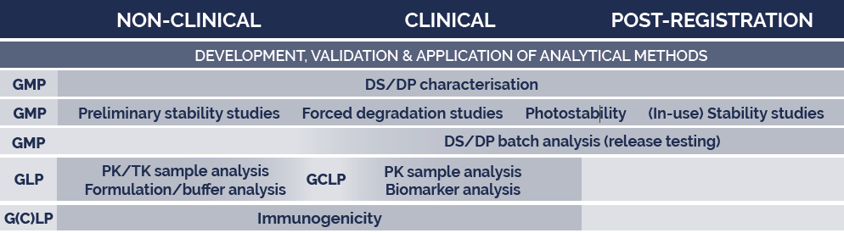
VACCINES
Whatever the type of antigen, Quality Assistance covers a wide range of analytical technologies needed to support vaccine development, including product characterisation, development and validation of analytical methods, stability studies, batch testing and bioanalytical services to support immunogenicity and protection studies.
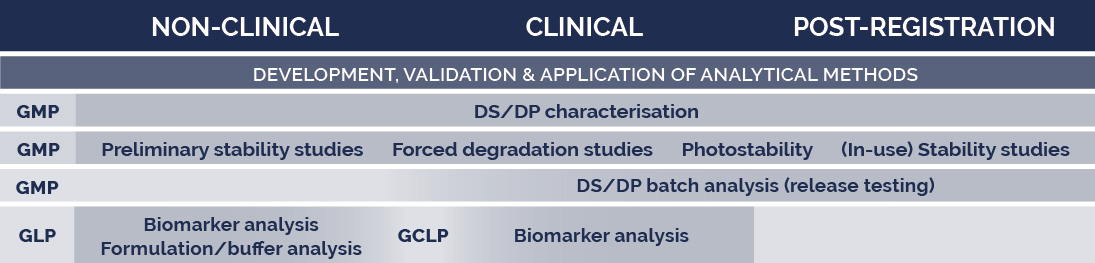
Don't hesitate to come and meet our team at the ESGCT Congress to discuss your outsourcing needs.
ABOUT THE EVENT
Visit https://www.esgctcongress.com/ for more information